Sterilization Requirements for Equipment in the Frozen Fish Industry
By. Najih - 20 Jun 2024
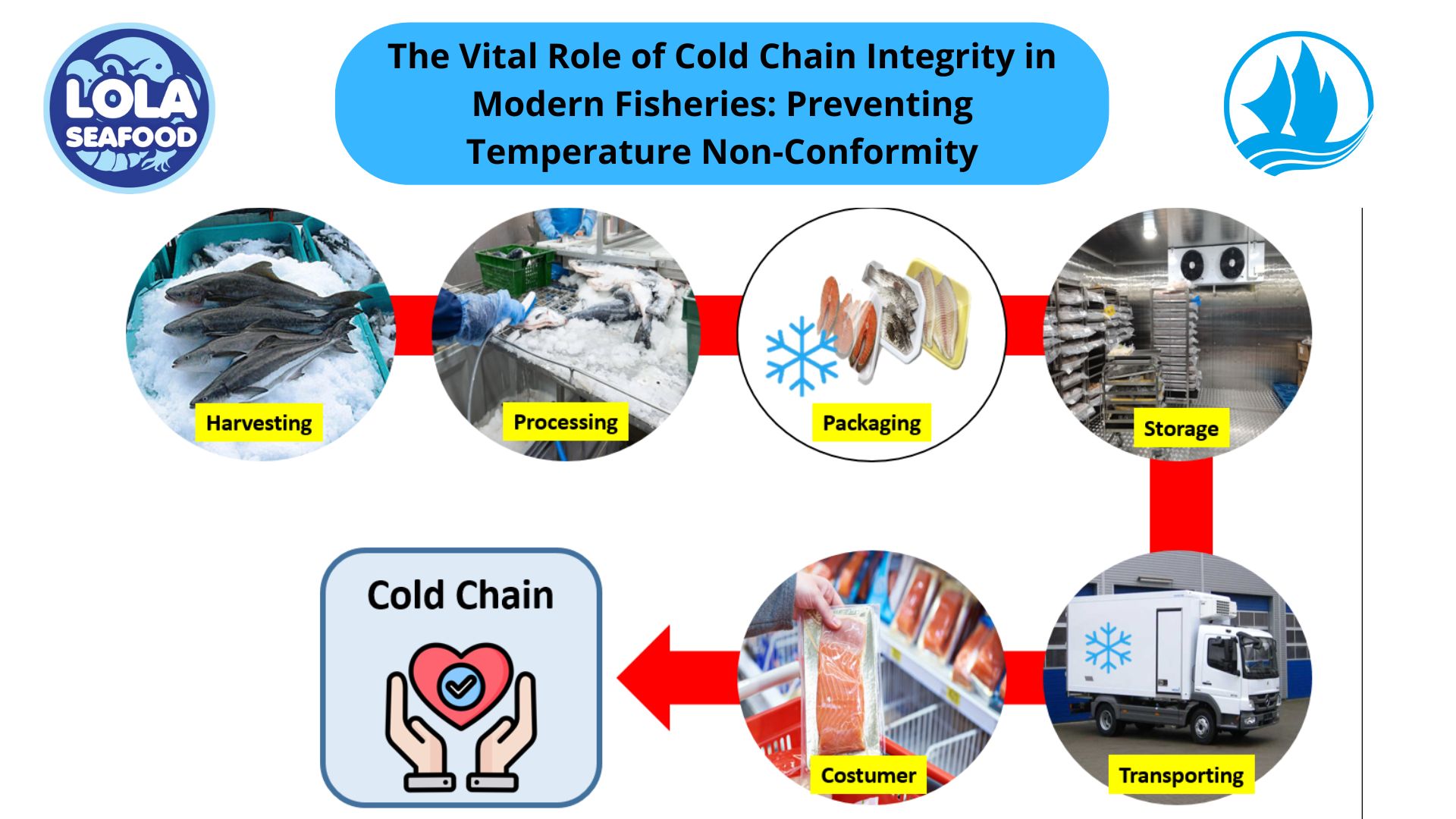
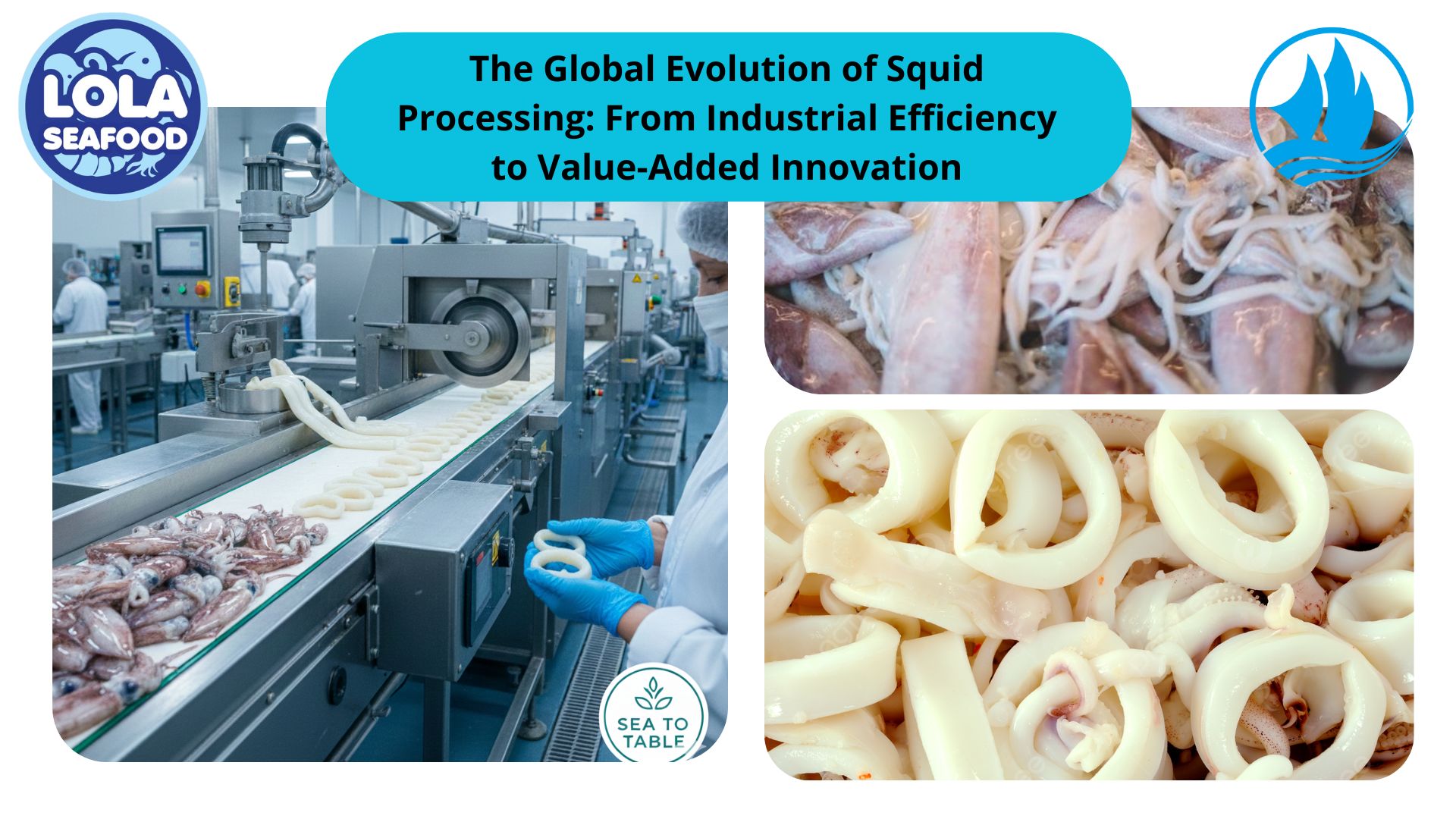
Sterilization of equipment is a critical aspect of ensuring food safety and quality in the frozen fish industry. Proper sterilization protocols are essential to prevent microbial contamination and maintain product integrity throughout processing. This article examines the sterilization requirements for equipment used in various stages of frozen fish processing, emphasizing the importance of adherence to stringent hygiene standards.
Sterilization Methods:
1. Heat-Based Sterilization: Heat treatments such as steam sterilization and hot water immersion are commonly used to sanitize equipment surfaces and kill pathogens. These methods effectively destroy microorganisms, including bacteria, viruses, and fungi, ensuring the hygienic conditions necessary for safe food processing.
2. Chemical Sterilization: Chemical agents such as chlorine, peracetic acid, and quaternary ammonium compounds are employed for disinfection purposes in the frozen fish industry. Proper dilution and contact time are crucial to ensure microbial reduction while minimizing chemical residues.
3. UV-C Sterilization: Ultraviolet-C (UV-C) radiation is utilized to disinfect equipment surfaces by damaging the DNA of microorganisms, thereby rendering them inactive. UV-C sterilization is effective against a wide range of pathogens and offers a chemical-free alternative for equipment sanitation.
4. Ozone Treatment: Ozone gas is a powerful oxidizing agent that can penetrate and destroy microbial cells, making it an effective sterilization method for equipment in the frozen fish industry. Ozone treatment not only eliminates pathogens but also helps control odors and reduce chemical usage.
5. Dry Heat Sterilization: Dry heat sterilization involves exposing equipment to elevated temperatures in the absence of moisture to achieve microbial inactivation. This method is suitable for heat-resistant materials and equipment components that cannot be steam sterilized.
Implementation and Monitoring:
1. Standard Operating Procedures (SOPs): Establishing comprehensive SOPs for equipment sterilization is essential to ensure consistency and efficacy in the sanitation process. SOPs should outline protocols for cleaning, disinfection, and sterilization, including frequency, methods, and verification procedures.
2. Validation and Verification: Regular validation and verification of sterilization processes are necessary to confirm their effectiveness in eliminating microbial contaminants. This may involve microbial testing, chemical concentration measurements, and visual inspections to assess equipment cleanliness.
3. Employee Training: Proper training of personnel responsible for equipment sterilization is crucial to minimize the risk of cross-contamination and ensure compliance with hygiene protocols. Training programs should cover proper cleaning techniques, chemical handling procedures, and personal hygiene practices.
.jpg)




.jpg)
.jpg)